搜索到 1000 条关于 럭스맘원본 Ap131.top 자갈치걸비디오 야동판 영천댁움짤 szi 的文章
-
2012.09.19 2011年生物仿制药会议(Biosimilars Asia 2011)
时间: 2011-5-23 至 2011-5-26
地点:Grand Hyatt Shanghai
主办单位: IBC Asia (S) Pte Ltd会议简介
According to Business Insights, an estimated $25bn worth of biologics will lose patent protection by 2016, creating a significant market opportunity for biosimilars. However, the reality is that biosimilar development is a risk-fraught activity that demands significant resources in R&D, manufacturing expertise, regulatory know-how, clinical-trial capabilities, and a high level of financial investment.
Biosimilar development is riddled with complexities, ranging from regulatory, to manufacturing to marketing, and is one of the most expensive propositions in the pharmaceutical industry. It is estimated that it costs between US$100-200 million to bring a biosimilar product to market. With so much at stake, biosimilars represent not only substantial growth but equally risk..
IBC’s 2nd Annual Biosimilars Asia 2011 Summit is the ONLY conference in Asia catering to this region’s specific queries. With unprecedented insights from leading innovators and biosimilar companies, you will network and learn how to strategically position your company to maximize your ROI. Biosimilars Asia 2011 is attended by key stakeholders in the region and beyond, enabling you to make informed strategic decisions.
5 Reasons To Attend - Only conference in Asia which addresses concerns faced by the Asian biosimilar companies with experts from the region and beyond
- Learn from and personally meet senior executives from the major stakeholders amongst from biosimilar to innovator companies
- The most comprehensive event highlighting key challenges in the industry including manufacturing, marketing, pricing, transitioning from generics to biosimilars and regulatory issues
- Showcasing unique case studies from leading Asian and international biosimilar companies
- Gather the latest insights from regional biosimilar companies and MNCs about their strategic decisions and how they are positioning themselves for success
Industry Gurus / Speakers Biocon, Pfizer, Genentech, Roche, Teva, Hospira, Sandoz, Momenta, Intas, Dr Reddy’s, Shenzhen Main Luck Pharmaceuticals Past Attendees Include Vice President, ASTRAZENECA INNOVATION CENTER (China)
Senior Director, GENENTECH INC, (USA)
Managing Director, BECTON DICKINSON CO (BD BIOSCIENCES) (Singapore)
Managing Director, ASAHI GLASS CO LTD (Japan)
Deputy General Manager, SINOPHARM (China)
Head, Biotech Research, USV LTD (India)
General Manager, JCR PHARMACEUTICALS CO LTD (Japan)
Vice President, R&D/Business Development, MAIN LUCK PHARMACEUTICALS INC (China)
Senior Research Associate, HANWHA CHEMICAL (South Korea)
Vice President, BOEHRINGER INGELHEIM GMBH (Germany)
General Manager, GLAXOSMITHKLINE (CHINA) INVESTMENT CO LTD (China)
IP Director, HOSPIRA (AUSTRALIA)
President, MYCENAX BIOTECH INC (Taiwan)
Senior Director, MERCK & CO INC (USA)
Associate Director, DR REDDY''''''''S LABORATORIES LTD (India)
Chairman, SHENZHEN SCIPROGEN BIO-PHARMACEUTICAL CO LTD (SCIPROGEN) (China)COO, SHANGHAI CELGEN BIOPHARMACEUTICALS (China)
Chief Representative, TTY BIOPHARM CO LTD (Taiwan)COO, 3SBIO (China)
Project Director, JIANGSU CHIA TAI TIANQING PHARMACEUTICAL CO LTD (China)Associate Director, PFIZER INVESTMENT CO LTD (China)
General Manager, NIPPON KAYAKU CO LTD (Japan)
President, HENLIUS BIOPHARMACEUTICALS INC & SHANGHAI HENLIUS BIOTECH (USA)
Vice President, RANBAXY (USA)Registration FEES per delegate Early Bird Rate
Register & Pay before 11 March 2011Special Rate
Register & Pay before 15 April 2011Normal Rate
Register & Pay after 15 April 2011Group Rate
Group of 3 or more? US$ / CNYUS$ / CNYUS$ / CNYUS$ / CNY4 day package
(Conf + Pre & Post Workshops)2,495 /
16,6002,695 /
17,9502,895 /
19,5002,175 /
14,6503.5 day package (Conf + Workshop C & A or B ) 2,295 /
15,5002,495 /
16,6002,695 /
17,9502,025 /
13,5003 day package (Conf + Workshop A & B or C) 1,995 /
13,5002,195 /
14,6002,395 /
15,9501,795 /
12,0002.5 day package (Conf + Workshop A or B) 1,795 /
12,0001,995 /
13,5002,195 /
14,6001,695 /
10,9502 day package
(Conf only)1,495 /
9,9501,695 /
11,3001,895 /
12,6001,495 /
9,950PAYMENT TERMS
Payment must be received 10 business days prior to the event. To take advantage of discounts with an expiry cut-off date, registration and payment must be received by the cut off date.Payments by Singapore Dollars (S$)
Payments by RMB (CNY)- Payments by S$ bank draft or cheque should be made in favour of “IBC Asia (S) Pte Ltd” payable in Singapore.
- Payment by telegraphic transfer in S$ must be made to:
IBC Asia (S) Pte Ltd
A/C No.: 147-059513-001 (SGD)
The Hongkong and Shanghai Banking Corporation Limited
21 Collyer Quay, HSBC Building, Singapore 049320
Bank Swift Code: HSBCSGSG
Bank Code: 7232 - Payment by Credit Card (AMEX, VISA or MASTERCARD). Please provide your Card Number, Name of Cardholder, Expiry Date and your Signature and send it by fax to +65 6508 2407.
- Payment by telegraphic transfer in CNY must be made to:
A/C Name:
A/C No.: 720-031103-001
Beneficiary Bank:
Bank Address: No. 1000 Lujiazui Rind Road,
Pudong, Shanghai 200120, P.R. China
Bank Swift Code: HSBCCNSH
Should you be unable to attend, a substitute delegate is welcome at no extra charge. Cancellations must be received in writing at least 10 business days before the start of the event, to receive a refund less 10% processing fee per registration. The company regrets that no refund will be made available for cancellation notifications received less than 10 business days before the event.IMPORTANT NOTE:
Please quote the name of the delegate, event title and invoice number on the advice when remitting payment. Bank charges are to be deducted from participating organisations own accounts. Please fax your payment details (copy of remittance advice, cheque or draft to +65 6508 2407.
Attendance will only be permitted upon receipt of full payment. Participants wishing to register at the door are responsible to ensure all details are as published. IBC Asia will not be responsible for any event re-scheduled or cancelled.
DATA PROTECTION
The personal information entered during your registration/order, or provided by you, will be held on a database and may be shared with companies in the Informa Group in the UK and internationally. Sometimes your details may be obtained from or shared with external companies for marketing purposes. If you do not wish your details to be used for this purpose, please contact Winnie Seah (Database) on winnie.seah@ibcasia.com.sg Tel: +65 6508 2468 or Fax: +65 6508 2408.联系方式电话:+65 650 82401
-

-
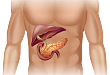
2020.05.27 【用药问答】急性胰腺炎的手术适应症为?
已出,评论区可回答延伸问题赢奖励)>>上期问答:变异型心绞痛临床用药首选?【延伸问题】急性胰腺炎内科治疗如何用药?答案:急诊之急性胰腺炎(AP
-
-
2016.11.23 不容忽视:这些药物易引起药源性胰腺炎
或继发微循环障碍破坏了胰腺间质细胞内的屏障防护作用 , 从而启动胰腺的自身消化机制 , 引发炎症。明确可引起药源性胰腺炎的药物包括:由于不同药物引起 AP 的机制不同, 即使同一药物对不同个体的致病过程和程度亦常有较大差异。目前 DAP 多为个例报道,因致病药物的类别和病人机体状况的差异, 不少人缺乏 AP 典型的腹痛
-

-
2016.03.16 梅奥新发现:C4d 可作为增生性肾小球肾炎诊断新工具
(AP)调节异常的结果。C3 肾小球病包括 C3 肾炎和致密物沉积病(DDD)。在补体介导 GN(C3 肾小球肾病)中,C3 染色明亮,Ig 染色通常阴性。然而... 例)C4d 染色痕量或 1+。至于感染后 GN,46%(6 例)的样本 C4d 染色阴性,提示 AP 途径不正常,54%(7 例)的样本阳性。(见图 1)图 1
-
2012.03.28 氯化两面针碱通过抑制c-Src/FAK相关信号通路抑制乳腺癌细胞的侵袭迁移
of RhoA, Rac1 and AP-1 transcriptional activity. Taken together, our results
-

-
2011.11.28 颈椎单开门扩大成形术后椎板会重新关闭
的前后(AP)径和椎板开门的角度。术前、术后1年通过MRI检查来评价脊髓压迫的严重程度。结果发现,椎管的前后径和椎板开门的角度虽在术后立即增加(均P
-

-
2021.08.02 2021 年 6 月最受欢迎的品牌案例
本期「TOP list ·月度案例评选」是基于 6 月在丁香园医院汇平台上发布的文章,根据阅读量及点赞量呈现医疗行业「最受欢迎的品牌案例」TOP 10。医院汇努力为医疗行业从业者打造优秀品牌案例库,供行业宣传人士参考学习,也是众医院打造品牌曝光的重要营地。新闻资讯 TOP 10排名医院
-

-
2020.06.30 2020 年 6 月最受欢迎的品牌案例
本期「TOP list ·月度案例评选」是基于 6 月在丁香园 医院汇 平台上发布的文章,根据阅读量及点赞量呈现医疗行业「最受欢迎的品牌案例」TOP 10。医院汇努力为医疗行业从业者打造优秀品牌案例库,供行业宣传人士参考学习,也是众医院打造品牌曝光的重要营地。新闻资讯 TOP 10
-

-
2015.11.14 丁香园力荐 最受医生欢迎的 20 本必备好书
「借花献佛」,和大家分享下推荐最多的 10 本 「案头必备」和 10 本 「床头必备」好书。一、案头必备TOP 1. 实用内科学推荐理由:中国医学界唯一一部畅销...出版一次,已出至第14版,可及时反映国内外进展,是内科领域各级学科医师的 「不二宝典」。TOP 2. 坎贝尔骨科手术学推荐理由:国际权威骨科经典著作,内含众多
-

-
2013.12.27 2013年5大重磅新药
2013年岁末已至,回首这一年,一批「重磅炸弹」级新药的诞生,使得生物制药成为全球医药领域的一个焦点。Top1 默克...晚期黑色素瘤临床试验和非小细胞肺癌试验结果提交给监管机构。Top2 糖尿病治疗的新突破强生和Evotec公司共同开发
-

-
2013.12.25 盘点2013年改变世界的5大重磅新药
2013年岁末已至,回首这一年,一批"重磅炸弹"级新药的诞生,使得生物制药成为全球医药领域的一个焦点。Top...积极的数据。制药巨头正准备将晚期黑色素瘤临床试验和非小细胞肺癌试验结果提交给监管机构。Top2:糖尿病治疗的新突破强生和Evotec公司
-

-
2017.08.29 嵇庆海教授专访:索拉非尼与放射性碘难治的分化型甲状腺癌
应对策略。ccvideo丁香园:您能否介绍一下目前分化型甲状腺癌的治疗现状?嵇庆海教授:分化型甲状腺癌是头颈部最常见的恶性肿瘤,以手术为主要治疗手段,辅之以碘-131 治疗和 TSH 抑制治疗。主要手术方式包括甲状腺腺叶切除、峡部切除,伴或不伴颈部淋巴结清扫等,对于中、高危患者,术后还要进行碘-131 治疗。但无论是否进行碘
-

-
2019.09.02 最新重症胰腺炎诊疗指南:合格的「摸金校尉」治疗篇
抗微生物药物治疗的正确疗程如何?预防性使用抗生素指南内容1. 最近的证据表明,AP 患者预防性使用抗生素并没有显著降低病死率或并发症发生率。因此,不再推荐对所有 AP 患者常规预防性使用抗生素(1A)。虽然早期的试验表明无菌性坏死的患者使用抗生素可能会预防感染性并发症,但后续的设计更好的试验
-

-
2014.03.11 标准不一,又何谈学术伦理?
ligase),可以催化它的 15 个长链氨基酸受体肽(amino acid acceptor peptide, AP)受质中的赖氨酸(Lysine)生物素化(biotinylation)反应。所以,生物素化的 AP 即可和含萤光的共轭链酶亲和素(streptavidin)结合,如此便可观测到活神经细胞表面交互作用的萤光
-
2012.09.19 2010 第五届国际基因组学大会(ICG-V)
, please click: http://www.genomeconference.org/pages/Abstract.aspxTop Poster
-
2020.06.17 【直播 | 现场】计算机辅助设计—分子模拟与蛋白互作研讨班(8.20-22)
。【报名回执】访问此链接填写报名回执(https://www.wjx.top/jq/80710449.aspx)【咨询请联系】QQ 号:2814500767邮箱
