搜索到 1000 条关于 p62카발디비업체문의)ㄱ텔darknessDB§ 的文章
-

-
2020.04.15 TAF联合TACE治疗HBV相关HCC病例
一、病例介绍• 患者 男 62 岁• 主诉:发现乙肝表面抗原阳性 16 年,腹胀伴下肢肿 20 天•
-

-
2019.03.28 NF-κB Signaling
点击下载 PDF>>通路描述:核因子-κB (NF-κB)/Rel 蛋白包括 NF-κB2 p52/p100、NF-κB1 p50/p105、c-Rel、RelA/p65 和 RelB。这些蛋白起到二聚化转录因子的作用,可调节基因表达,并可影响到各种不同的生物学过程,包括先天和适应性免疫、炎症、应激反应
-

-
2018.07.13 你问我答23期:β- 内酰胺类能否合用地塞米松?
本期问答:@dxy_aio5fn62运用头孢的患者能不能使用激素类的注射剂,因为胃肠外科病人术前会预防用头孢,而全麻时麻醉医生会忽略病人是否用头孢,习惯性运用地塞米松,泼尼松等,是否会发生双硫仑反应。参考答案:丁香园站友 @wangshuping :β- 内酰胺类和地塞米松不要一起使用,原因是:1、β- 内酰胺
-
2012.09.19 首届中国PBPK模拟软件GastroPlus Workshop第二轮通知
首届中国PBPK模拟软件GastroPlus Workshop第二轮通知
北京·2012年5月16-18日
上海·2012年5月21-23日尊敬的老师,您好!生理药代动力学模型(PBPK)在药物研发与监管中得到广泛应用意味着新时代的来临!它改变了动物试验和人体实验传统研究模式流程,指导制药企业项目决策及效率提高;FDA和EMA的使用及推荐,促使国外制药企业及学术单位相关研究者都在学习及掌握PBPK的应用。为促进我国在该领域的发展,力争与全球同步,凡默谷特邀美国Simulations Plus顶尖专家来华授课,让您了解及掌握PBPK知识、获悉国外最新应用、亲授多年使用经验,授课过程将以理论与实践相结合的形式进行。GastroPlus是由美国Simulations Plus公司研发的基于机制性生理模型的药代动力学、药效动力学(PBPK/PD)模拟软件,目前在FDA和几乎所有的全球顶尖制药公司中得到广泛应用,被誉为同类软件中的“黄金标准”。为确保培训效果,限额50人/班,第一轮通知发出后,已得到广大中国用户的积极响应和踊跃报名。报名从速!为何本次GastroPlus Workshop值得您参加?1)药物研发及监管中争相应用PBPK模型的时代已经来临!2)PBPK辅助药物研究可方便指导项目决策、节约成本、缩短时间;3)FDA已广泛采用PBPK模拟来制定法规和对新药及仿制药进行审批;4)FDA及各大制药企业对全球最权威的PBPK模拟软件GastroPlus倍加青睐;5)零距离接触Simulations Plus首席科学家,并参与学习的最新应用案例;6)您可免费获得一个月的GastroPlus软件试用。本次GastroPlus Workshop将让您受益良多:1)制药企业或CRO:有利于您理解您的客户或其他Sites实验方案设计思路、下一阶段决策及实验开展、合理设计实验方案;2)学术研究:启示您开辟新的药代、药剂等研究方向,剖析药物作用机制等;3)临床药理研究:合理设计临床试验方案,不同人群PK/PD研究等;4)法规部门:协助制定新药及仿制药政策和标准。 报名方法:请您填写报名回执,并通过Email的方式反馈给我们。Email: training@pharmogo.com报名截止:2012年4月20日(北京培训班)/2012年4月30日(上海培训班)联系人:周伟,电话:021-61638583-8001报名确认:1)报名确认以凡默谷收到款项或报名者提供汇款凭证为准,凡默谷可先提供发票以供报销;2)会务组在接到报名后,将email或电话通知学员培训班相关事项。温馨提醒:学员必须自带已安装好Gastroplus程序的电脑参加培训,现场不提供电脑。
GastroPlus Workshop Course Instructors List

Michael B. Bolger
Chief Scientist
Simulations Plus Inc.
Dr. Bolger is Chief Scientist at Simulations Plus, he oversees a team of scientist / programmers at Simulations Plus, Inc. (Lancaster, CA) in the development of software programs (ADMET Predictor/Modeler, MedChem Studio/Designer, GastroPlus, DDDPlus, and MembranePlus) for estimation of biopharmaceutical properties, simulations of absorption and bioavailability, automated generation QSP/AR model building, in vitro dissolution, and cell culture permeability simulation.
As a faculty member at USC School of Pharmacy, his research was supported by several NIH basic science research grants and he published over 50 peer-reviewed publications. From 1989-1991 he served as a FDA National Science Advisor. He has served as a reviewer for numerous scientific publications. He is currently Adjunct Associate Professor of Pharmaceutical Sciences at USC.

Haiying Zhou, Ph.D.
Senior Scientist II
Simulations Plus Inc.
Dr. Zhou is Senior Scientist II at Simulations Plus, she focused on the development of modeling and simulation software for quantitative understanding of the pharmacokinetics and pharmacodynamics of drug candidates (GastroPlus), in vitro dissolution of active pharmaceutical ingredients and formulation excipients under various experimental conditions (DDDPlus). She received her M.S. degree in automation from East China University of Science and Technology (ECUST) in 2002 and her Ph.D in Biomedical Engineering from Case Western Reserve University (CWRU) in 2009. Her research interests in CWRU include multi-scale mathematical modeling of gas transport and cellular metabolism with emphasis on the metabolic regulation during different conditions, e.g. hypoxia and exercise.

John DiBella
Vice President of Marketing Simulations Plus Inc.
John DiBella is the Director – Marketing & Sales at Simulations Plus, He joined Simulations Plus in 2003 and has spent time working on the development of GastroPlus? and DDDPlus? software and consulting projects. Now, he leads the marketing & sales activities for the company while continuing to travel the world hosting training seminars.John obtained B.S. and M.S. degrees (with honors) in Biomedical Engineering from Case Western Reserve University in 2003. His graduate research was focused on computational modeling of physiological systems. 培训班详情:请下载附件参加北京培训班请点击下载:GastroPlus北京培训班第二轮通知/BeiJing GastroPlus-Intro-Workshop 2nd Notice参加北京培训班请点击下载:GastroPlus上海培训班第二轮通知/ShangHai GastroPlus-Intro-Workshop 2nd Notice
-
2012.09.19 首届中国PBPK模拟软件GastroPlus Workshop第二轮通知
首届中国PBPK模拟软件GastroPlus Workshop第二轮通知
北京·2012年5月16-18日
上海·2012年5月21-23日尊敬的老师,您好!生理药代动力学模型(PBPK)在药物研发与监管中得到广泛应用意味着新时代的来临!它改变了动物试验和人体实验传统研究模式流程,指导制药企业项目决策及效率提高;FDA和EMA的使用及推荐,促使国外制药企业及学术单位相关研究者都在学习及掌握PBPK的应用。为促进我国在该领域的发展,力争与全球同步,凡默谷特邀美国Simulations Plus顶尖专家来华授课,让您了解及掌握PBPK知识、获悉国外最新应用、亲授多年使用经验,授课过程将以理论与实践相结合的形式进行。GastroPlus是由美国Simulations Plus公司研发的基于机制性生理模型的药代动力学、药效动力学(PBPK/PD)模拟软件,目前在FDA和几乎所有的全球顶尖制药公司中得到广泛应用,被誉为同类软件中的“黄金标准”。为确保培训效果,限额50人/班,第一轮通知发出后,已得到广大中国用户的积极响应和踊跃报名。报名从速!为何本次GastroPlus Workshop值得您参加?1)药物研发及监管中争相应用PBPK模型的时代已经来临!2)PBPK辅助药物研究可方便指导项目决策、节约成本、缩短时间;3)FDA已广泛采用PBPK模拟来制定法规和对新药及仿制药进行审批;4)FDA及各大制药企业对全球最权威的PBPK模拟软件GastroPlus倍加青睐;5)零距离接触Simulations Plus首席科学家,并参与学习的最新应用案例;6)您可免费获得一个月的GastroPlus软件试用。本次GastroPlus Workshop将让您受益良多:1)制药企业或CRO:有利于您理解您的客户或其他Sites实验方案设计思路、下一阶段决策及实验开展、合理设计实验方案;2)学术研究:启示您开辟新的药代、药剂等研究方向,剖析药物作用机制等;3)临床药理研究:合理设计临床试验方案,不同人群PK/PD研究等;4)法规部门:协助制定新药及仿制药政策和标准。 报名方法:请您填写报名回执,并通过Email的方式反馈给我们。Email: training@pharmogo.com报名截止:2012年4月20日(北京培训班)/2012年4月30日(上海培训班)联系人:周伟,电话:021-61638583-8001报名确认:1)报名确认以凡默谷收到款项或报名者提供汇款凭证为准,凡默谷可先提供发票以供报销;2)会务组在接到报名后,将email或电话通知学员培训班相关事项。温馨提醒:学员必须自带已安装好Gastroplus程序的电脑参加培训,现场不提供电脑。
GastroPlus Workshop Course Instructors List

Michael B. Bolger
Chief Scientist
Simulations Plus Inc.
Dr. Bolger is Chief Scientist at Simulations Plus, he oversees a team of scientist / programmers at Simulations Plus, Inc. (Lancaster, CA) in the development of software programs (ADMET Predictor/Modeler, MedChem Studio/Designer, GastroPlus, DDDPlus, and MembranePlus) for estimation of biopharmaceutical properties, simulations of absorption and bioavailability, automated generation QSP/AR model building, in vitro dissolution, and cell culture permeability simulation.
As a faculty member at USC School of Pharmacy, his research was supported by several NIH basic science research grants and he published over 50 peer-reviewed publications. From 1989-1991 he served as a FDA National Science Advisor. He has served as a reviewer for numerous scientific publications. He is currently Adjunct Associate Professor of Pharmaceutical Sciences at USC.

Haiying Zhou, Ph.D.
Senior Scientist II
Simulations Plus Inc.
Dr. Zhou is Senior Scientist II at Simulations Plus, she focused on the development of modeling and simulation software for quantitative understanding of the pharmacokinetics and pharmacodynamics of drug candidates (GastroPlus), in vitro dissolution of active pharmaceutical ingredients and formulation excipients under various experimental conditions (DDDPlus). She received her M.S. degree in automation from East China University of Science and Technology (ECUST) in 2002 and her Ph.D in Biomedical Engineering from Case Western Reserve University (CWRU) in 2009. Her research interests in CWRU include multi-scale mathematical modeling of gas transport and cellular metabolism with emphasis on the metabolic regulation during different conditions, e.g. hypoxia and exercise.

John DiBella
Vice President of Marketing Simulations Plus Inc.
John DiBella is the Director – Marketing & Sales at Simulations Plus, He joined Simulations Plus in 2003 and has spent time working on the development of GastroPlus? and DDDPlus? software and consulting projects. Now, he leads the marketing & sales activities for the company while continuing to travel the world hosting training seminars.John obtained B.S. and M.S. degrees (with honors) in Biomedical Engineering from Case Western Reserve University in 2003. His graduate research was focused on computational modeling of physiological systems. 培训班详情:请下载附件参加北京培训班请点击下载:GastroPlus北京培训班第二轮通知/BeiJing GastroPlus-Intro-Workshop 2nd Notice参加北京培训班请点击下载:GastroPlus上海培训班第二轮通知/ShangHai GastroPlus-Intro-Workshop 2nd Notice
-

-
2012.11.22 依那西普治疗RA继发AA淀粉样变优于环磷酰胺
月9日的Rheumatology杂志上。研究认为,对于RA继发AA淀粉样变,依那西普治疗比环磷酰胺更有效。有62位和24位RA患者分别接受环磷酰胺...,以及两种治疗方法的有效性对比。接受ETN治疗的患者入组时似肾功能更差,如24尿蛋白更高(P=0.02)。与CYC相比,ETN治疗在血清CRP和血
-
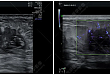
-
2021.09.02 【高尚病例】浅表超声—乳腺癌
病史摘要患者女性、62 岁,因「发现左侧乳腺肿块几月余」来本...(肿瘤表达具有异质性,热点区 70%+),HER2(阴性,积分 1+),Ki67(70%+),GATA-3(+),SMMHC 及 P63(示肌上皮缺失,E-Cad
-
2022.07.20 2022 年浙江大学发表 20 项 NCS 研究成果
。CCT2 与不依赖于货物泛素化的易聚集蛋白相关联,并通过非经典 VLIR 基序与自噬体标志物 ATG8 相互作用。此外,CCT2 独立于泛素结合受体(P62、NBR1 和 TAX1BP1)或伴侣介导的自噬调节聚集体自噬。与促进具有流动性的蛋白质凝聚物的清除的 P62、NBR1 和 TAX1BP1 不同,CCT2 专门促进
-

-
2014.07.23 海南334支医疗防疫队伍自备饮食救灾
截止23日,海南全省共派出62支医疗队和272支消毒防疫队伍,自备饮食前往受超级台风“威马逊”影响的灾区救灾。记者从海南省卫生和计划生育委员会了解到。22日...地区灾后医疗支援,海南省皮肤病医院、海南省妇幼保健院、海南省眼科医院每日派队流动巡诊。目前,共有62支医疗队343名医疗人员在灾区参与医疗救治。海南省卫计委要求
-

-
2012.08.16 支架内新形成内膜的动脉粥样硬化一例
光学相干断层扫描图像一名植入“全金属外套”后15年患者支架内晚期血栓的原因:使用光学相干断层扫描的观察结果62岁男性患者因急性前壁ST段抬高性急性心肌梗死行直接PCI术。患者15年有心肌梗死病史,右冠内植入金属裸支架。从图像上看,冠脉造影提示右冠全程支架内完全闭塞
-

-
2013.08.08 泉州滨海医院
泉州滨海医院
-

-
2013.07.06 福建医科大学招聘会现场
院校招聘会
